GRAY SLENDER LORISES
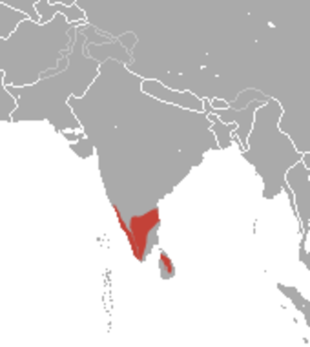
Gray slender lorises (Loris lydekkerianus) live in tropical areas in forests, rainforests and scrub forests and agricultural areas in southern India. They were once classified as Slender lorises lydekkerianus, a subspecies of slender lorises, which now scientists say lives only in Sri Lanka. In 2001, the taxonomy of lorises was updated based on behavioral, geographic, and morphological data. Gray slender lorises are now considered a separate slender loris species found in India and Sri Lanka. Both slender loris species are unique among the members of the family Lorisidae in many respects. The species are unusually social, sleeping in groups and regularly interacting with other individuals during nighttime foraging. The species also occasionally exhibit fast locomotion, which has not been observed in other species of Lorisidae. Lastly, the slender loris species are uniquely gregarious, emitting loud contact calls throughout the night.[Source: Abi Nishimura, Animal Diversity Web (ADW) /=]
Gray slender lorises survive in a wide variety of habitats. Within these habitats, the ideal environment for these largely tree-dwelling creatures has species contains plentiful oblique and horizontal surfaces for climbing, feeding, and mating. These animals are also found in many ecological zones including wet zones, low dry zones, and low country zones. The range of Gray slender lorises have been expanded to an unknown extent due to the careless collection and distribution of plant materials. Gray slender lorises are sometimes inadvertently transported with plant materials. /=\
Gray slender lorises are classified as Least Concern on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species due to its “wide distribution, tolerance of a broad range of habitats, presumed large population, and because it is unlikely to be declining fast enough to qualify for listing in a more threatened category” . In CITES (Convention on International Trade in Endangered Species of Wild) they are in Appendix II, which lists species not necessarily threatened with extinction now but that may become so unless trade is closely controlled. Threats include habitat destruction, particularly of primary and secondary forest and the reduction of forest cover caused by mainly the use of forest land for agriculture. Fragmented forest patches impede the movements and success of the Gray slender loris. The main known predators are humans, spotted owlets and domestic cats. Lorises are also threatened by hunting and trapping for use in traditional medicine. Gray slender lorises has been observed reacting to potential predators by emitting loud calls, fleeing, or maintaining a large distance from the threat.
RELATED ARTICLES:
LORISES factsanddetails.com
SLENDER LORISES factsanddetails.com
SLOW LORISES factsanddetails.com
PYGMY SLOW LORISES factsanddetails.com ;
PRIMATES: HISTORY, TAXONOMY, CHARACTERISTICS, BEHAVIOR factsanddetails.com ;
MAMMALS: HAIR, CHARACTERISTICS, WARM-BLOODEDNESS factsanddetails.com
Gray Slender Loris Characteristics
Gray slender lorises range in weight from 180 to 290 grams (6.3 to 10.2 ounces). Sexual Dimorphism (differences between males and females) is present: Males are larger than females. The weight of males ranges from 180 to 290 grams, depending on the subspecies while female weight ranges from 180 to 275 grams. The average length of males is 24.1 centimeters (inches), while that of females is 23.4centimeters. [Source: Abi Nishimura, Animal Diversity Web (ADW) /=]
Gray slender lorises have grey or reddish dorsal fur with a darker medial stripe and a white ventrum. Their distinctly forward facing eyes are large and set closely together, while the rostrum (hard, beak-like structures projecting out from the head or mouth) is small and pointed. Abi Nishimura wrote in Animal Diversity Web: The coloring of the face is also distinctive; they have circumocular patches, darker preauricular hair, and a white rim between the circumocular patch and preauricular hair. Gray slender lorises have no tails and the limbs are long and extremely slim.
Gray slender lorises have many distinctive derived characteristics including extremely slender limbs, the closest orbital approximation of all primates, small hands in comparison with the feet, feet with shortened second digits, a unique non-saltatory locomotor style, digestive specializations for ingesting toxic prey, and an unusually low basal metabolic rate. The species also exhibits retia mirabilia of the proximal limb vessels, an adaptation that allows for extended periods of arboreal (live mainly in trees), clinging. /=\
Gray slender loris appearance changes significantly throughout its development. Infants (4 to eight weeks) have fluffy, large heads relative to body size. Juveniles (2 to three months) have particularly fluffy fur all over the body, and adults (4 months onward) exhibit full body size and complete adult coloration patterns.
Gray Slender Loris Subspecies
There are four gray slender loris subspecies, that differ in geographic location, fur, and size: 1) Highland slender loris(Loris lydekkerianus grandis); 2)Mysore slender loris (Loris lydekkerianus lydekkerianus); 3)Malabar slender loris (Loris lydekkerianus malabaricus); and 4) Northern Ceylonese slender loris (Loris lydekkerianus nordicus).
Mysore slender lorises have gray fur, a narrow circumocular patch, and a broad white rim between the dark preauricular hair and circumocular patch. The subspecies is relatively larger, with males weighing approximately 260 grams and females weighing 275 grams. The head length, body length, and head breadth of the Mysore slender loris is larger than lorises than in Malabar slender lorises. Malabar slender lorises have a reddish body color, a broad circumocular patch, and a narrow white rim between the dark preauricular hair and circumocular patch. Both male and female adults weigh approximately 180 grams.
Northern Ceylonese slender lorises and Highland slender lorises are listed as Endangered on the International Union for Conservation of Nature (IUCN) Red List, while Malabar slender lorises malabaricus and Gray slender lorises lydekkerianus are listed as Near Threatened. Mysore slender lorises and Malabar slender lorises have more sustainable numbers. Malabar slender lorises are found throughout the Western Ghats in a contiguous population.
Gray Slender Loris Food, Eating and Hunting
Gray slender lorises are almost entirely insectivorous (eat mainly insects). More than half of their diet is composed of ants and termites. Theu lorises also consume a large variety of other arthropods, including other insects, such as beetles and orthopterans, spiders, mollusks, and occasional small vertebrates such as amphibians and reptiles, In captivity, slender lorises consume a variety of small animals including insects, small mammals, and geckos. [Source: Abi Nishimura, Animal Diversity Web (ADW) /=]
According to Animal Diversity Web: Many prey species contain toxic chemicals. The consumption of toxic species is accompanied by an elaborate behavioral repertoire including sneezing, head shaking, sucking of the hands and feet, and urine-washing. Rarely, individuals eat tree gum by scraping the surface of hardened tree gum with their toothcomb (a group of teeth with fine spaces between them)s to access the soft exudate beneath.
Prey is detected primarily by vision and smell. The most common hunting behavior involves visual or olfactory detection, ear retraction, noticeable sniffing, and a meticulously slow approach. Most frequently, one hand holds onto a substrate while the other hand hovers near the prey before quickly darting forward and grabbing the prey in a sudden burst of motion. Occasionally, these lorises catch prey bimanually or will directly consume prey with the mouth. One individual was observed repeatedly allowing termites to climb onto its saliva-coated hand, trapping them in the saliva before licking them off. Large prey are eaten head-first and any wings are typically removed before consumption. The majority of feeding events involve food items found in patches such as insect aggregations. This pattern has implications about the unusually gregarious and social nature of the species. Gray slender lorises are most frequently found near trees with heavy orthopteran leaf damage and near trees associated with ant colonies. Additionally, population density is positively correlated with insect density.
Gray Slender Loris Behavior
Gray slender lorises are arboreal (live mainly in trees), scansorial (able to or good at climbing), nocturnal (active at night), motile (move around as opposed to being stationary), sedentary (remain in the same area), territorial (defend an area within the home range), and social (associates with others of its species; forms social groups). The size of their range territory is 1.12 to 3.6 hectares. The range of adult males is 2.36 to 3.6 hectares, with a central, core range size of 0.37 hectares. The range of adult females is 1.12 to 1.59 hectares, with a core area size of 0.15 hectares. Abi Nishimura wrote in Animal Diversity Web: Juvenile males have an average home range size of 1.17 hectares. Until 10 months old, young gray slender lorises remain within their mother’s home range. Female adults have little intrasex overlap in their home ranges, while males have large intrasex overlap. Any intrasex range overlap is limited to peripheral range areas, and core areas remain exclusive to one individual. Intersex range overlap is common and can include core areas. A single male’s home range can overlap with the home ranges of multiple females. The average density within gray slender loris ranges is 2.4 lorises per square kilometers. [Source:Abi Nishimura, Animal Diversity Web (ADW) /=]
Other loris species are almost completely solitary, but Gray slender lorises and slender lorises are unique in their regular social interactions. During the day, gray slender lorises almost always sleep in social groups of two to seven individuals that are most frequently composed of an adult female, her offspring, and a small number of adult or subadult males. The location and composition of these sleeping groups are generally constant. The sleeping site is typically centrally located within the home range of the primary female. Sleeping sites are generally located in cacti or tangled branches. Individuals sleeping together usually form a “sleeping ball” congregation in which individuals tangle their limbs together. At dusk, the sleeping site members wake and groom one another, with grooming occurring between individuals of all ages and sexes. Behavioral data about sleeping site composition and home range patterns supports the hypothesis that slender lorises have a multi-male social system.
During nighttime foraging, gray slender lorises are generally solitary, though amicable foraging pairs have been observed. Males and subadult males often visit parked infants during the night. In general, social interactions occur between adult males and females, as well as between adults and juveniles, but rarely occur between adults of the same sex. Gray slender lorises and the slender lorises spend up to half of their time in the presence of other individuals. Research on activity budget has also shown that the species spends approximately 45 percent of its time engaging in inactive behaviors such as sitting, vigilant watching, resting, and sleeping. The rest of the activity budget is spent foraging or traveling and a small percentage is spent grooming. The most common positional behaviors are sitting and quadrupedal (use all four limbs for walking and running) movement. A slow, crypic, non-saltatory locomotor pattern is considered characteristic of the family Lorisidae. However, the slender lorises are the only species within the family that have also been observed moving quickly; the slender loris species have been seen running and engaging in short jumps, though these kinds of fast movements are rare. The generally slow speed and silent motions protect the species against detection by predators that rely on visual or auditory cues. For this reason, particularly slow movements are used while moving across open ground between discontinuous substrates. The slow locomotor strategy also aids in food acquisition; particularly slow movements are used immediately before catching fast moving insect prey.
Gray Slender Loris Communication
Gray slender lorises sense communicate with vision, touch, sound and chemicals usually detected by smelling and scent marks produced by special glands and placed so others can smell and taste them. Unlike most members of the family Lorisidae, gray slender lorises are relatively gregarious. The species maintains social networks with frequent loud calls throughout the night. Loud calls are also used when potential predators are detected, during reproduction, and during infant care-taking. [Source: Abi Nishimura, Animal Diversity Web (ADW) /=]
Males interested in estrous females use more frequent vocalizations while following females. Male-male competition also includes complex growling, chittering, and whistling. Females emit these same noises when chasing away unwanted suitors. Additionally, parked infants will emit “zic" sounds approximately thirty minutes before dawn to alert the mothers of their location. Micturition, or urination, is another important method of communication.
The species uses rhythmic micturition and urine washing as methods of territorial (defend an area within the home range), olfactory marking. Urine washing has also been observed as a stress response. Such olfactory behaviors are used for social communication. Slow lorises have keen low-light vision because of their nocturnal (active at night),ity. Prey is detected primarily by vision and smell.
Gray Slender Loris Mating and Reproduction
Gray slender lorises are polygynandrous (promiscuous), with both males and females having multiple partners. Females mate with multiple males within a single estrous cycle and can mate with several males in a row. Males mate with multiple females throughout the year. The female estrous cycle lasts for 24 hours. External genitalia is present in adults, with estrous females displaying enlarged genitalia, and male testes alternating between descended and inguinal stages every other night. In Gray slender lorises, no pattern has been observed with respect to male testes state and sexual activity. However, in the closely related slender loris, enlargement of male genitals appears to be affected primarily by ambient temperature, with testes enlargement occurring during periods of increased temperature. [Source: Abi Nishimura, Animal Diversity Web (ADW) /=]
Gray slender lorises engage in year-round breeding and breed as often as once every 5.5 months. Peak birth times occur during April and May and from October to December. The number of offspring is one or two, with both occurring with equal frequency. Females have two sets of nipples, a feature that proves useful when females give birth to twins.The reproductive seasonality of gray slender lorises are disputed. Some researchers claim that the most frequent estrous periods occur biannually, in April to June and October to December. Others claim that births are distributed throughout the year, and that any apparent reproductive peaks are simply a result of the 5.5 month gestation length.
According to Animal Diversity Web: Gray slender lorises are reported to form social associations in which the larger home ranges of one or more males overlap the smaller range of a single female. The presence of a post-copulation vaginal plug of hardened semen has been reported. This, along with elaborate penile morphology, has been taken as evidence of sperm competition and a multi-male breeding system. Nekaris observed several wild male Gray slender lorises rotating among three estrus females, with each male separately grooming each female at different times over the course of a single night. Nekaris proposes a multi-male, multi-female (promiscuous) mating system. [Source: Rory McGuinness, Animal Diversity Web (ADW) /=]
Males display interest in estrous females by increasing grooming frequency, emitting more frequent vocalizations, and by following the female. Males follow foraging females for long periods of time, anywhere from one to dozens of hours. During this period, females can physically deter the trailing males with physical cuffs. Male-male competition can occur between males trailing the same female. These confrontations involve vocalizations such as growling, chittering, and whistling. Male-male competition can also be physically violent involving chasing and grappling. The most successful male competitor is normally permitted mating privileges by the female.
If a female permits mating, copulation occurs with the female suspended on a horizontal branch. Single mating intromissions last anywhere from three to 11 minutes. A complete sexual encounter often consists of several individual mating intromissions, and can last for up to 12 hours. Depending on the outcomes of male-male competition, different males can mate with the same female consecutively. Studies of captive animals show that male ejaculation is associated with male pelvis wiggling and the insertion of a “copulatory plug” into the female that serves to block the mating success of other males. Females have been observed removing and consuming the copulatory plug.
Mating in Northern Ceylonese slender lorises can last for five hours and is characterized by appeasement vocalizations and excited branch-shaking on the part of the male, and threat vocalizations by the female. In captive Gray slender lorises, when the female is ready for copulation she communicates her acceptance by hanging using all four limbs. Intromission lasts from two to sixteen minutes and is concluded by a threat vocalization from the female. Both genders lick their genitals after copulation. A single copulation of Gray slender lorises has been documented in the wild, which was preceded by an hour-long courtship pursuit and occurred in a suspensory position under a horizontal branch. Males in other trees harassed the mating pair, and copulation was twice interrupted while the focal male chased away his rivals.
Gray Slender Loris Offspring and Parenting
The average gestation period of gray slender loriseis is 5.5 months. The interbirth interval lasts approximately seven months. This relatively high reproductive potential may be due to male allocare and the high-energy milk provided by the mother. The average weaning age is five months and the average time to independence is four months. Females reach sexual or reproductive maturity at 10 to 15 months and males reach sexual or reproductive maturity at age 10 months. [Source: Abi Nishimura, Animal Diversity Web (ADW) /=]
Parental care is provided by females. Males provide allocare but only to infants that share their sleeping sites, The genetic relationship between these males and infants is unknown. Female allocare is rare; females almost exclusively care for their own infants in the form of feeding, carrying, grooming, and protection.
According to Animal Diversity Web: Mothers groom infants exhaustively for the first three days after birth, and after this period groom only upon infant vocalization. Mothers constantly carry their infants during the first four weeks of life. At four weeks, infants are ‘parked’ near the sleeping site at night while the mother forages.
The timeline of infant development is largely shaped by the ‘parking’ behavior demonstrated by females. For the first four weeks of life, infants are carried all the time by the mother. Approximately four weeks after birth, mothers begin to ‘park’ infants at night before leaving to forage. The exact timing of the onset of this parking behavior is likely related to the parenting experience of the mother. While infants are parked, mothers almost never return until dawn. During this time, males and subadult males often visit parked infants, sometimes grooming or playing with the infants.
Image Sources: Wikimedia Commons
Text Sources: Animal Diversity Web animaldiversity.org ; National Geographic, Live Science, Natural History magazine, David Attenborough books, New York Times, Washington Post, Los Angeles Times, Smithsonian magazine, Discover magazine, The New Yorker, Time, Reuters, Associated Press, AFP, Lonely Planet Guides, Wikipedia, The Guardian, Top Secret Animal Attack Files website and various books and other publications.
Last updated December 2024