OCEAN ACIDIFICATION

Oa-sami, ocean acid
measuring devise Ocean acidification refers to a reduction in the pH of the ocean over an extended period of time, caused primarily by uptake of carbon dioxide (CO2) from the atmosphere. For more than 200 years, or since the industrial revolution, the concentration of carbon dioxide (CO2) in the atmosphere has increased due to the burning of fossil fuels and land use change. The ocean absorbs about 30 percent of the CO2 that is released in the atmosphere, and as levels of atmospheric CO2 increase, so do the levels in the ocean. [Source: National Oceanic and Atmospheric Administration (NOAA)]
In some places scientists have observed rises in acidity of 30 percent and predict 100 to 150 percent increases by 2100. The mixture of carbon dioxide and seawater creates carbonic acid, the weak acid in carbonated drinks. The increased acidity reduces the abundance of carbonate ions and other chemicals necessary to form calcium carbonate used make sea shells and coral skeletons. To get an idea what acid can due to shells remember back to high school chemistry classes when acid was added to calcium carbonate, causing it to fizz.
Ocean acidification is affecting the entire world’s oceans, including coastal estuaries and waterways. Many economies are dependent on fish and shellfish and people worldwide rely on food from the ocean as their primary source of protein. The acidification that has occurred so far is probably irreversible. Although in theory it's possible to add chemicals to the sea to counter the effects of the extra CO2, as a practical matter, the volumes involved would be staggering; it would take at least two tons of lime, for example, to offset a single ton of carbon dioxide, and the world now emits more than 30 billion tons of CO2 each year. Meanwhile, natural processes that could counter acidification — such as the weathering of rocks on land — operate far too slowly to make a difference on a human time-scale. Even if CO2 emissions were somehow to cease today, it would take tens of thousands of years for ocean chemistry to return to its pre-industrial condition.
Related Articles:
HUMANS AND THE OCEANS: MYTHS, PRODUCTS, ETIQUETTE AND DRUGS ioa.factsanddetails.com
POLLUTION IN THE SEA ioa.factsanddetails.com
RED TIDES (HARMFUL ALGAL BLOOMS): DEAD ZONES, EUTROPHICATION, CAUSES AND IMPACTS ioa.factsanddetails.com
OIL IN THE OCEAN: SPILLS, NATURAL SEEPAGE AND SHIPS ioa.factsanddetails.com
GLOBAL WARMING AND THE SEA ioa.factsanddetails.com
ENDANGERED CORAL REEFS AND THEIR RECOVERY AND REBIRTH ioa.factsanddetails.com
GLOBAL WARMING AND CORAL REEFS ioa.factsanddetails.com
CORAL BLEACHING: CAUSES, CONSEQUENCES AND RECOVERY ioa.factsanddetails.com
HELPING CORAL RECOVER AND REVIVE ioa.factsanddetails.com
RECOMMENDED BOOKS:
“Climate and the Oceans” (Princeton Primers in Climate) by Geoffrey K. Vallis Amazon.com
“The Encyclopedia of Weather and Climate Change: A Complete Visual Guide”
by Juliane L. Fry, Hans-F Graf, et al. Amazon.com
“Atmosphere, Ocean and Climate Dynamics: An Introductory Text” (International Geophysics by John Marshall, R. Alan Plumb Amazon.com
“Great Ocean Conveyor: Discovering the Trigger for Abrupt Climate Change”
by Wally Broecker Amazon.com
“Beyond Extinction: The Eternal Ocean―Climate Change & the Continuity of Life”
by Wolfgang Grulke Amazon.com
“The Unnatural History of the Sea” by Callum Roberts (Island Press (2009) Amazon.com
“The Ocean of Life: The Fate of Man and the Sea” by Callum Roberts Amazon.com
“Plastic Soup: An Atlas of Ocean Pollution” by Michiel Roscam Abbing Amazon.com
“Blue Hope: Exploring and Caring for Earth's Magnificent Ocean” by Sylvia Earle (2014) Amazon.com
“The Empty Ocean” by Richard Ellis (2003) Amazon.com
“Oceans: The Threats to Our Seas and What You Can Do to Turn the Tide” by , Jon Bowermaster (2010) Amazon.com
“Dark Side of The Ocean: The Destruction of Our Seas, Why It Maters, and What We Can Do About It” by Albert Bates Amazon.com
“Ocean's End: Travels Through Endangered Seas” (2001)
by Colin Woodard Amazon.com
“The Blue Machine: How the Ocean Works” by Helen Czerski, explains how the ocean influences our world and how it functions. Amazon.com
“The Science of the Ocean: The Secrets of the Seas Revealed” by DK (2020) Amazon.com
“Atmospheric and Oceanic Fluid Dynamics: Fundamentals and Large-Scale Circulation” by Geoffrey K. Vallis (2006) Amazon.com
“Essentials of Oceanography” by Alam Trujillo and Harold Thurman Amazon.com
“Ocean: The World's Last Wilderness Revealed” by Robert Dinwiddie , Philip Eales, et al. (2008) Amazon.com
Chemistry of Ocean Acidification
Extra carbon dioxide molecule in sea water alters the pH level of sea water, making it slightly more acidic. When CO2 is absorbed by seawater, a series of chemical reactions occur resulting in the increased concentration of hydrogen ions. This increase causes the seawater to become more acidic and causes carbonate ions to be relatively less abundant.
Elizabeth Kolbert wrote in National Geographic, The pH scale, which measures acidity in terms of the concentration of hydrogen ions, runs from zero to 14. At the low end of the scale are strong acids, such as hydrochloric acid, that release hydrogen readily (more readily than carbonic acid does). At the high end are strong bases such as lye. Pure, distilled water has a pH of 7, which is neutral. Seawater should be slightly basic, with a pH around 8.2 near the sea surface. So far CO2 emissions have reduced the pH there by about 0.1. Like the Richter scale, the pH scale is logarithmic, so even small numerical changes represent large effects. A pH drop of 0.1 means the water has become 30 percent more acidic. If present trends continue, surface pH will drop to around 7.8 by 2100. At that point the water will be 150 percent more acidic than it was in 1800. [Source: Elizabeth Kolbert, National Geographic, April 2011]
Ocean Acidification May Increase Global Warming
More acidic oceans may produce less a gas that is essential to cooling the Earth. Allie Bidwell wrote in U.S. News and World Report: As oceans become more acidic by soaking up carbon dioxide released into the atmosphere, they will produce less of a gas that protects the Earth from the sun's radiation and will amplify global warming, according to a new study from a team of German researchers. It is a widely held belief that carbon dioxide emissions contribute to global warming, and that oceans lessen the effects of those emissions by absorbing a large amount of carbon dioxide. But the study, says past research has not linked climate change and ocean acidification. [Source: Allie Bidwell, U.S. News and World Report, August 26, 2013]
Scientists at the Max Planck Institute for Meteorology in Germany found that concentrations of the compound dimethyl sulfide (DMS), which produces the gas that helps cool the Earth's surface, were significantly lower in waters with higher levels of acidity. The lower a solution's pH level is, the more acidic it is considered. Over the last 200 years, oceans have absorbed about 525 billion tons, roughly 50 percent of human-released carbon dioxide emissions. "The 'price' for storing [carbon dioxide] is an ongoing decrease of seawater pH, a process that is likely to have diverse and harmful impacts on marine biota, food webs, and ecosystems," the study says.
Changes in the acidity of oceans, and thus the amount of DMS they produce, have the potential "to notably alter the Earth's radiation budget," the study says. Historically, sea water has had a pH level of around 8.16, but has fallen by about 0.1 units since the beginning of the Industrial Revolution, to 8.05, according to the National Oceanic and Atmospheric Administration's Pacific Marine Environmental Laboratory. Although that sounds like a small change, it represents a nearly 30 percent increase in acidity, according to the Center for Ocean Solutions, located in Monterey, Calif. Oceans have not seen a change so "abrupt and large" for at least 650,000 years, the center says. Research predicts that sea water pH levels may continue to decrease by as much as 0.4 units by 2100.
And that's also a problem because higher acidity levels will adversely affect different ocean species that many people rely on for food and jobs, such as oysters, clams, sea urchins and deep sea corals, according to NOAA. "Today, more than a billion people worldwide rely on food from the ocean as their primary source of protein," NOAA's Pacific Marine Environmental Laboratory says. "Many jobs and economies in the U.S. and around the world depend on the fish and shellfish in our oceans."
Affects of Ocean Acidification on Sea Life

Acid-affected pteropod Carbonate ions are an important building block of structures such as sea shells and coral skeletons. Decreases in carbonate ions can make building and maintaining shells and other calcium carbonate structures difficult for calcifying organisms such as oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton. These changes in ocean chemistry can affect the behavior of non-calcifying organisms as well. Certain fish's ability to detect predators is decreased in more acidic waters. When these organisms are at risk, the entire food web may also be at risk.
Elizabeth Kolbert wrote in National Geographic, “Acidification has myriad effects. By favoring some marine microbes over others, it is likely to alter the availability of key nutrients like iron and nitrogen. For similar reasons it may let more sunlight penetrate the sea surface. By changing the basic chemistry of seawater, acidification is also expected to reduce the water's ability to absorb and muffle low-frequency sound by up to 40 percent, making some parts of the ocean noisier. Finally, acidification interferes with reproduction in some species and with the ability of others — the so-called calcifiers — to form shells and stony skeletons of calcium carbonate. These last effects are the best documented ones, but whether they will prove the most significant in the long run is unclear. “[Source: Elizabeth Kolbert, National Geographic, April 2011]
High acidity makes it difficult for some species of mollusks, gastropods and corals to produce their shells and poisons the acid-sensitive eggs of some species of fish such as amberjack and halibut. If populations of these organisms collapse then populations of fish and other creatures that feed on them could also suffer.
Elizabeth Kolbert wrote in National Geographic, “Meanwhile, scientists are just beginning to explore the way that ocean acidification will affect more-complex organisms such as fish and marine mammals. Changes at the bottom of the marine food web — to shell-forming pteropods, say, or coccolithophores — will inevitably affect the animals higher up. But altering oceanic pH is also likely to have a direct impact on their physiology. Researchers in Australia have found, for example, that young clownfish — the real-life versions of Nemo — can't find their way to suitable habitat when CO2 is elevated. Apparently the acidified water impairs their sense of smell. [Source: Elizabeth Kolbert, National Geographic, April 2011]
Ocean acidification may pose a risk to red king crab and other lucrative fisheries in Alaska, a new report says. According to research by NOAA published in the online journal Progress in Oceanography. changes in ocean chemistry could make it harder for mollusks and other small creatures to build and keep their skeletons or shells. Previous studies have shown red king crab and tanner crab grow more slowly in more acidic water and that red king crab died in highly acidified conditions. [Source: Becky Bohrer, Associated Press, July 30, 2014]
Study Says Fish Losing Survival Instinct in Acidic Oceans
Fish are losing their survival instinct — even becoming attracted to the smell of their predators — as the world's oceans become more acidic because of climate change according to a study published in 2014. AFP reported: The study of fish in coral reefs off the coast of Papua New Guinea — where the waters are naturally acidic — showed the animals' behaviour became riskier. "Fish will normally avoid the smell of a predator, that makes perfect sense," lead author Professor Philip Munday from Australia's James Cook University told AFP. "But they start to become attracted to the smell of the predator. That's incredible. They also swim further from shelter and they are more active, they swim around more. That's riskier behaviour for them — they are more likely to be attacked by a predator." [Source: AFP, April 14, 2014]
Munday said the research, published in the journal Nature Climate Change, was important given that about 30 percent of the carbon dioxide released into the atmosphere is ultimately absorbed by the ocean, a process which results in the seas becoming more acidic. Acidification around the reefs studied is at levels predicted to become ocean-wide by the end of the century as the climate changes.
Munday said the fish appeared to have failed to adapt to the conditions, despite living their whole lives exposed to high levels of carbon dioxide. "They didn't seem to adjust within their lifetime," Munday said. "That tells us that they don't adjust when they are permanently exposed to these higher carbon dioxide levels and we would have to think about whether adaptation would be possible over the coming decades."
Munday said the "seep" to which the fish were exposed — in which carbon dioxide from undersea volcanic activity bubbles to the surface — was the perfect "natural laboratory" for the study. Close to the seep there is no coral growth, but further away lies a unique coral reef zone with carbon dioxide levels similar to those forecast for future decades. Co-author Jodie Rummer said while the increased carbon dioxide in the water affected how fish behaved, it did not appear to affect their athletic performance. "The metabolic rates of fish from the seep area were the same as fish from nearby 'healthy' reefs," she said in a statement. "So, it seems that future ocean acidification may affect the behaviour of reef fishes more than other aspects of their performance."
History of Ocean Acidification — It’s the Rate That Matters
Elizabeth Kolbert wrote in National Geographic: “During the long history of life on Earth, atmospheric carbon dioxide levels have often been higher than they are today. But only very rarely — if ever — have they risen as quickly as right now. For life in the oceans, it's probably the rate of change that matters. [Source: Elizabeth Kolbert, National Geographic, April 2011]
“To find a period analogous to the present, you have to go back at least 55 million years, to what's known as the Paleocene-Eocene Thermal Maximum or PETM. During the PETM huge quantities of carbon were released into the atmosphere, from where, no one is quite sure. Temperatures around the world soared by around ten degrees Fahrenheit, and marine chemistry changed dramatically. The ocean depths became so corrosive that in many places shells stopped piling up on the seafloor and simply dissolved. In sediment cores the period shows up as a layer of red clay sandwiched between two white layers of calcium carbonate. Many deepwater species of foraminifera went extinct.

shells that experienced
heavy acidification “Surprisingly, though, most organisms that live near the sea surface seem to have come through the PETM just fine. Perhaps marine life is more resilient than the results from places like Castello Aragonese and One Tree Island seem to indicate. Or perhaps the PETM, while extreme, was not as extreme as what's happening today.
“The sediment record doesn't reveal how fast the PETM carbon release occurred. But modeling studies suggest it took place over thousands of years — slow enough for the chemical effects to spread through the entire ocean to its depths. Today's rate of emissions seems to be roughly ten times as fast, and there's not enough time for the water layers to mix. In the coming century acidification will be concentrated near the surface, where most marine calcifiers and all tropical corals reside. "What we're doing now is quite geologically special," says climate scientist Andy Ridgwell of the University of Bristol, who has modeled the PETM ocean.
In Waters Where Ocean Acidification Is Already Taking Place
Elizabeth Kolbert wrote in National Geographic, “Owing to a quirk of geology, the sea around Castello Aragonese — a tiny island in the Tyrrhenian Sea 17 miles west of Naples — provides a window onto the oceans of 2050 and beyond. Bubbles of CO2 rise from volcanic vents on the seafloor and dissolve to form carbonic acid. Carbonic acid is relatively weak; people drink it all the time in carbonated beverages. But if enough of it forms, it makes seawater corrosive. "When you get to the extremely high CO2, almost nothing can tolerate that," Jason Hall-Spencer, a marine biologist from Britain's University of Plymouth, explains. Castello Aragonese offers a natural analogue for an unnatural process: The acidification that has taken place off its shore is occurring more gradually across the world's oceans, as they absorb more and more of the carbon dioxide that's coming from tailpipes and smokestacks. [Source: Elizabeth Kolbert, National Geographic, April 2011]
Hall-Spencer has been studying the sea around the island for the past eight years, carefully measuring the properties of the water and tracking the fish and corals and mollusks that live and, in some cases, dissolve there. On a chilly winter's day I went swimming with him to see the effects of acidification up close. We anchored our boat about 50 yards from the southern shore of Castello Aragonese. Even before we got into the water, some impacts were evident. Clumps of barnacles formed a whitish band at the base of the island's wave-battered cliffs. "Barnacles are really tough," Hall-Spencer observed. In the areas where the water was most acidified, though, they were missing.
Searching for food, some limpets had wandered into water that was too caustic for them. Their shells were so thin they were almost transparent. Bubbles of carbon dioxide streamed up from the seafloor like beads of quicksilver. We swam on. Beds of sea grass waved beneath us. The grass was a vivid green; the tiny organisms that usually coat the blades, dulling their color, were all missing. Sea urchins, commonplace away from the vents, were also absent; they can't tolerate even moderately acidified water. Swarms of nearly transparent jellyfish floated by. "Watch out," Hall-Spencer warned. "They sting."
Jellyfish, sea grass, and algae — not much else lives near the densest concentration of vents at Castello Aragonese. Even a few hundred yards away, many native species can't survive. The water there is about as acidified as the oceans as a whole are forecast to be by 2100. "Normally in a polluted harbor you've got just a few species that are weedlike and able to cope with widely fluctuating conditions," Hall-Spencer said once we were back on the boat. "Well, it's like that when you ramp up CO2."
Affects of Ocean Acidification on Coral Reefs
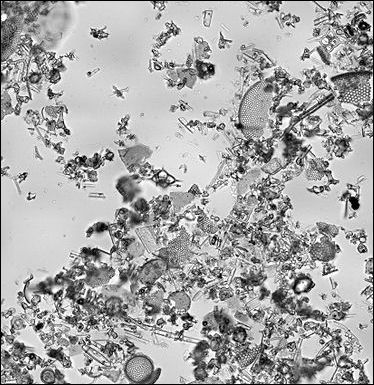
diatom walls broken down by acids
Elizabeth Kolbert wrote in National Geographic, “Ocean acidification adds yet another threat, one that may be less immediate but ultimately more devastating to hard, reef-building corals. It undermines their basic, ancient structure — the stony skeleton that's secreted by millions upon millions of coral polyps over thousands of years....To make calcium carbonate, corals need two ingredients: calcium ions and carbonate ions. Acids react with carbonate ions, in effect tying them up. So as atmospheric CO2 levels rise, carbonate ions become scarcer in the water, and corals have to expend more energy to collect them. Under lab conditions coral skeleton growth has been shown to decline pretty much linearly as the carbonate concentration drops off. [Source: Elizabeth Kolbert, National Geographic, April 2011]
Slow growth may not matter much in the lab. Out in the ocean, though, reefs are constantly being picked at by other organisms, both large and small. (When I went snorkeling off One Tree Island, I could hear parrotfish chomping away at the reef.) "A reef is like a city," said Ove Hoegh-Guldberg, who used to direct the One Tree Island Research Station and now heads the Global Change Institute at Australia's University of Queensland. "You've got construction firms and you've got demolition firms. By restricting the building materials that go to the construction firms, you tip the balance toward destruction, which is going on all the time, even on a healthy reef. In the end you wind up with a city that destroys itself."
See Separate Article CORAL BLEACHING AND GLOBAL WARMING'S IMPACT ON REEFS factsanddetails.com
Affects of Ocean Acidification on Calcium-Producing Organisms
Elizabeth Kolbert wrote in National Geographic, “Corals, of course, are just one kind of calcifier. There are thousands of others. Crustaceans like barnacles are calcifiers, and so are echinoderms like sea stars and sea urchins and mollusks like clams and oysters. Coralline algae — minute organisms that produce what looks like a coating of pink or lilac paint — are also calcifiers. Their calcium carbonate secretions help cement coral reefs together, but they're also found elsewhere — on sea grass at Castello Aragonese, for instance. It was their absence from the grass near the volcanic vents that made it look so green. [Source: Elizabeth Kolbert, National Geographic, April 2011]
The seas are filled with one-celled calcifying plants called coccolithophores, whose seasonal blooms turn thousands of square miles of ocean a milky hue. Many species of planktonic foraminifera — also one-celled — are calcifiers; their dead shells drift down to the ocean floor in what's been described as a never ending rain. Calcifiers are so plentiful they've changed the Earth's geology. England's White Cliffs of Dover, for example, are the remains of countless ancient calcifiers that piled up during the Cretaceous period.
Acidification makes all calcifiers work harder, though some seem better able to cope. In experiments on 18 species belonging to different taxonomic groups, researchers at the Woods Hole Oceanographic Institution found that while a majority calcified less when CO2 was high, some calcified more. One species — blue mussels — showed no change, no matter how acidified the water.
"Organisms make choices," explained Ulf Riebesell, a biological oceanographer at the Leibniz Institute of Marine Sciences in Kiel, Germany. "They sense the change in their environment, and some of them have the ability to compensate. They just have to invest more energy into calcification. They choose, 'OK, I'll invest less in reproduction' or 'I'll invest less in growth.'" What drives such choices, and whether they're viable over the long term, is not known; most studies so far have been performed on creatures living for a brief time in tanks, without other species that might compete with them. "If I invest less in growth or in reproduction," Riebesell went on, "does it mean that somebody else who does not have to make this choice, because they are not calcifying, will win out and take my spot?"

Ocean Acidification Weakens the Shells of Sea Snails
There are concerns that global warming could deplete the oceans of calcifying plankton, including small snails call pteropods. These small creatures (usually about 0.3 centimeters in size) are a critical part of the chain in polar and near polar seas. They are a favorite food of herring, pollock, cod, salmon and whales. Large masses of them are a sign of a healthy environment. Research has shown that their shells dissolve when placed in water acidified by carbon dioxide. Shells with large amounts of the mineral aragonote — a very soluble form of calcium carbonate — are particularly vulnerable. Pteropods are such creatures, In one experiment a transparent shell was placed in water with the amount of dissolved carbon dioxide expected to be in the Antarctic Ocean by the year 2100. After just two days the shell becomes pitted and opaque. After 15 days it becomes badly deformed and had all but disappeared by day 45.
The shells of some marine snails in the seas around Antarctica are dissolving as the water becomes more acidic, threatening the food chain, a study published in the journal Nature Geoscience said. Reuters reported: The shell of the pteropod sea snail in the Southern Ocean was severely dissolved by more acidic surface water, the researchers from the British Antarctic Survey, Royal Netherlands Institute for Sea Research, the U.S. National Oceanic and Atmospheric Administration (NOAA) and other institutions found. And although the snails did not necessarily die, it increased their vulnerability to predators and infection which could affect other parts of the food chain. “The corrosive properties of the water caused shells of live animals to be severely dissolved and this demonstrates how vulnerable pteropods are,” said lead author Nina Bednaršek, from the NOAA. [Source: Nina Chestney, Reuters, November 26, 2012]
“Ocean acidification, resulting from the addition of human-induced carbon dioxide, contributed to this dissolution.” The sea snails are an important source of food for fish and birds as well as an indicator of marine ecosystem health. But until now, there has been little evidence of the impact of ocean acidification on such live organisms in their natural environment and the study supports predictions that acidification could have a significant effect on marine ecosystems. The researchers examined surface water, where wind causes cold water to be pushed up from deeper water, because it is usually more corrosive to a particular type of calcium carbonate which the sea snails use to build and maintain their shells.
“We know that the seawater becomes more corrosive ... below a certain depth which occurs at around 1,000m. However, at one of our sampling sites, we discovered that this point was reached at 200m depth. Marine snails — pteropods — live in this top layer of the ocean,” Bednaršek said.
Ocean Acidification May Have Triggered Mass Extinctions 252 Million Years Ago
The biggest extinction known on Earth — the mass extinction at the boundary of the Permian and Triassic eras 252 million years ago --- was caused, scientists believe, by the acidification of the world’s oceans. Tim Radford wrote in Climate News Network: “The Permian Extinction — sometimes called “the Great Dying” — seemed to all but obliterate life in the oceans, and perhaps on land. More than 90 percent of all species disappeared, more than 80 percent of all genera, and more than 50 percent of all marine families were extinguished in one prolonged calamity. All life on Earth today has descended from the few survivors of this far-off episode. Palaeontologists, geologists, climate scientists and astronomers have all speculated on the probable cause. [Source: Tim Radford, Climate News Network, April 18, 2015]

Ocean acidity: Aragonite saturation state is commonly used to track ocean acidification because it is a measure of carbonate ion concentration. When aragonite saturation state falls below 3, these organisms become stressed, and when saturation state is less than 1, shells and other aragonite structures begin to dissolve.
“The latest and most confident analysis is based on a new study of ancient marine sediments and delivers obvious parallels with processes that are — for different reasons — occurring again today. Matthew Clarkson of the University of Edinburgh in Scotland (but now at the University of Otago in New Zealand) and colleagues report in the journal Science that they examined limestone from the United Arab Emirates and found, in the isotope ratios of the element boron, evidence of ocean acidity in carbonate rocks that were laid down as sediment at the bottom of the ocean 250 million years ago.
“A change in the isotope ratios, they calculated, would have indicated a significant shift in seawater chemistry. Over the last 40 years, researchers have introduced a whole suite of plausible triggers for the Permian extinction, but at last one team had clear evidence of increased atmospheric carbon, probably from a prolonged and convulsive series of volcanic eruptions that gave rise to vast, ancient geological formations now known as the Siberian Traps. “Scientists have long suspected that an ocean acidification event occurred during the greatest mass extinction of all time, but direct evidence has been lacking until now”, said Dr Clarkson. “This is a worrying finding, considering that we can already see an increase in ocean acidity today that is the result of human carbon emissions.”
“There has been recent evidence that this present change in the pH of ocean waters (pH is a measure of its acidity) as a consequence of fossil fuel combustion in the last two centuries has already disturbed the behaviour of some fish species, threatened to affect oyster fisheries and coral reefs, and even to alter whole ocean ecosystems.
“The changes in the Permian were not sudden: ecosystems already seriously under stress because of lack of oxygen or rising temperatures were then dramatically affected by discharges of carbon dioxide that were probably much greater than all the modern world’s existing fossil fuel reserves could deliver. As the oceans became more acidic, many species were extinguished forever: among them the trilobites. The whole chain of events took 60,000 years. Humans have been burning fossil fuels for only 200 years, but, the researchers point out, in the Permian crisis, carbon was probably being released into the atmosphere at the rate of about 2.4 billion tons a year. Right now, humans are estimated to be releasing carbon from fossil fuels at the rate of 10 billion tons a year.
Image Source: National Oceanic and Atmospheric Administration (NOAA) noaa.gov/ocean and Wikimedia Commons, except giant jellyfish from Hector Garcia blog
Text Sources: Mostly NOAA, National Geographic articles. Also the New York Times, Washington Post, Los Angeles Times, Smithsonian magazine, Natural History magazine, Discover magazine, Times of London, The New Yorker, Time, Newsweek, Reuters, AP, AFP, Lonely Planet Guides, Compton’s Encyclopedia and various books and other publications.
Last updated February 2023
